

Gastric gastric balloon applications, which are used in the treatment of obesity disease, are being used more and more day by day. The fact that the application is reversible, involves much less risk and complications compared to surgical operations, and is an ideal method for patients who have problems with surgery. The state-of-the-art swallowable gastric balloon Allurion Elipse Gastric balloon, developed in parallel with technological developments, does not require endoscopy and anesthesia like traditional endoscopic gastric balloons. Many studies have been conducted on the safety and effectiveness of gastric balloons. The following article has been translated from the source text as a summary of the most comprehensive study worldwide on the ellipse gastric balloon to date.
Source: PMCID: PMC7458897 | PMID: 32279182 National Library of Medicine
Obesity disease can now be described as a worldwide epidemic. Diet and exercise are ineffective in controlling this epidemic. Bariatric surgery, although effective, has significant risks. Minimally invasive techniques, such as endoscopic gastric balloons, are being developed to provide a safer alternative for weight loss. The new swallowable gastric balloon, Elipse® (Allurion Technologies, Natick, MA, USA), respresents an innovative option for weight loss that does not require endoscopy or anesthesia. Several previous studies on the Elipse balloon have shown it to be a simple, safe and effective method for weight loss [1-5]. Although the residence time of the elliptical swallowable gastric balloon is shorter than that of endoscopic intragastric balloons, the results obtained at the end of the procedure seem comparable to others [1-3]. In addition, the minimal risk nature of the Elipse method may provide a new treatment modality for multiple patient categories. Overweight patients who cannot choose a riskier treatment may choose Elipse, and patients with higher BMI who are afraid of anesthesia may have more than one Elipse balloon treatment in a row. The efficacy of sequential endoscopic intragastric balloon treatments in morbidly obese patients has already been reported in several studies [6–9]. Recently, the efficacy of using endoscopic intragastric gastric balloons and swallowable gastric balloons as a treatment in obese adolescents and young ages has been determined by studies [10, 11]. The Elipse gastric balloon may represent a more usable tool for weight loss in this difficult-to-manage patient category. In addition, the easier application of the Elipse balloon and the absence of endoscopy for insertion or removal allow its use to become widespread not only for the surgeon, but also for other obesity specialists.
The Allurion Elipse Gastric Balloon is compressed into a swallowable vegan capsule attached to a thin catheter in which the balloon is filled (Figure 1). 550 mL of liquid is filled with a catheter after the capsule is placed in the stomach and its location is checked with x-ray. Placement is performed in a 20-minute outpatient visit without endoscopy or sedation. After filling and by abdominal X-ray, the correct position of the balloon is confirmed, the thin catheter is withdrawn and removed (Fig. 2). At approximately 4 months, the valve in the Elipse gastric balloon opens spontaneously (Figure 3), the balloon empties and is then excreted through the gastrointestinal tract. In case of swallowing difficulties, a thin guide wire can be used, which acts as a stylet (to gently push the catheter).
Figure 1: Elipse Swallowable Gastric Balloon
Figure 2: Elipse Balloon Key Innovations
Figure 3: Elipse Balloon Valve Mechanism
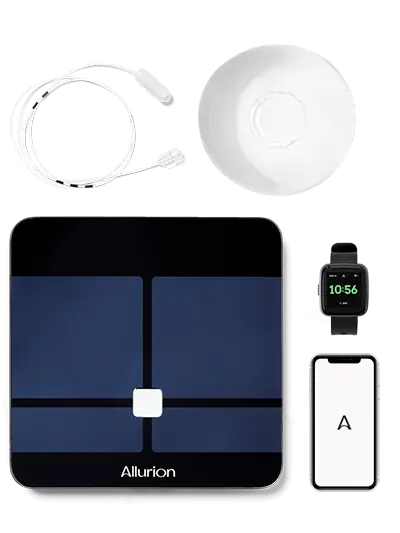
The Elipse balloon is accompanied by a wireless, Bluetooth enabled body composition scale and a smartphone application that enable weight loss tracking and communication between the patient and his/her care team (Figure 4).
Figure 4: Allurion Elipse Weight Loss Program Equipment (Smart scale, smart watch, smart phone app)
Elipse swallowable gastric balloon is a new treatment option for weight loss that does not require endoscopy and anesthesia. The capsule containing the balloon is swallowed with water and filled with liquid in the stomach and inflated. After 4 months, the balloon empties by itself and is expelled naturally. The purpose of the source study was to evaluate the safety and efficacy of the Elipse balloon in a large, multicenter population.
In this context, Elipse swallowable gastric balloon was applied to a total of 1770 patients in 19 centers around the world. Data from 1770 Elipse gastric balloon patients were analyzed. Data included weight loss, metabolic parameters, ease of insertion, balloon performance, and complications.
Weight loss (WL)(kg): Weight loss is calculated as 4th month weight (kg) – starting weight (kg).
Percentage total body weight loss (%TBWL): %TBWL is calculated as [(Month 4 weight (kg) − Initial weight (kg))/(Basic weight (kg)] × 100%. (Total Body Weight Loss)
BMI (BMI Loss) loss (kg/m2): BMI loss is calculated as 4th Month BMI (kg/m2) – Initial BMI (kg/m2). (Body Mass Index)
Percent excess weight loss (%EBWL): %EBWL is calculated as {[(Base BMI-25) − (Month 4 BMI-25)]/(Base BMI-25)} using a reference normal BMI of 25 kg/m2. × 100%. (Excess Body Weight Loss)
Change in laboratory values: The difference between Month 4 and Baseline was calculated for each laboratory value of triglycerides (mg/dL), LDL (mg/dL), and HbA1c (%).
19 international obesity centers were included in the study, which was conducted in multiple clinics worldwide, covering a total of 1770 overweight and obese (1264 Female 506 Male) patients from January 2016 to June 2019. Data collection started in January 2016. Data were collected at each site by predefined personnel with experience in data collection. After it was sent to the author coordinator, he combined all the data for data analysis. Inclusion criteria were 18 to 65 years of age and a body mass index (BMI) greater than 27 kg/m2 with previous failed dietary treatments. Pregnant women, patients with a history of three or more cesarean sections, patients with swallowing problems, patients with pre-existing bowel obstruction, patients with bulky hiatal hernia, and patients with GI cancer were excluded. Also, GI bleeding, severe coagulopathy or severe psychological or eating disorders, conditions predisposing to intestinal obstruction (history of perforated appendicitis; history of abdominal or pelvic surgery performed at least 12 months prior to treatment for Ellipse: diagnostic laparoscopy, laparoscopic appendectomy, with right lower quadrant incision open appendectomy, laparoscopic cholecystectomy, inflammatory bowel disease, ulcerative colitis; severe GI motility disorder such as severe gastroparesis); and patients with conditions predisposing to gastric perforation (history of previous gastric or esophageal surgery; previous history of laparoscopic band ligation; history of anti-reflux surgery) were excluded from the study.
To prevent the increase of gastroesophageal reflux discomfort, patients begin prophylactic treatment with PPI 2 weeks before insertion and continue this treatment for 4 months during which the balloon remains in the stomach. Depending on the intensity of reflux symptoms reported by any patient at the time of the screening visit, each doctor may consider the option of performing an endoscopy prior to balloon placement to assess the complete condition of the esophagus and stomach. Endoscopy or imaging was performed to evaluate if there were any symptoms suggestive of a gastroesophageal problem, such as abdominal pain, persistent or severe reflux, and abdominal tenderness.
During the Elipse research program, patients were closely followed by a dedicated multidisciplinary team. The program started 2 weeks before balloon placement and continued until balloon passage in approximately 4 months. A detailed medical obesity history, feeding behavior history, and anthropometric evaluation (height, weight, BMI, waist circumference) were performed before placement. Lab values were pooled on a subset of sites. These regions were chosen because of their strong interest in obesity-related metabolic disorders. Four hours prior to balloon insertion, patients received a single dose (125 mg PO) of anti-emetic aprepitant (Emend®). Immediately after balloon insertion, patients were given ondansetron 4 mg PO every 8 hours for 3 days, with two more doses of aprepitant (80 mg PO) and an anti-spasmodic prescribed as needed before discharge. Patients were treated daily with a proton pump inhibitor (lansoprazole 30 mg/day PO or equivalent PPI) for the entire treatment period, starting 2 weeks prior to balloon insertion, to improve the safety of the gastric balloon, if present, to improve any asymptomatic superficial inflammation. Patients were advised to avoid NSAIDs (Non-steroidal anti-inflammatory drugs) and other stomach irritants during the study. Patients were fasted for at least 8 hours before the balloon insertion procedure. Only liquid hydration was allowed for the first 24 hours and a gradual progression to a semi-solid diet followed by a solid diet was followed by the patients for 1-2 weeks. The diets were administered by a nutritionist or dietitian who supported the patients throughout the entire treatment period. All patients received a wireless, Bluetooth®-enabled body composition scale and a smartphone app (Figure 1), which allows weight loss tracking and communication between patient and care team. Monthly face-to-face visits were made until the end of the program.
Of the 1770 patients who participated in the study, 1264 were female and 506 were male. 63 patients (3.6%) failed to complete the program and had the balloon removed before 4 months due to intolerance or other adverse events.
At the beginning of the study, the profile of the patients was determined as follows: Mean age 38.8±12 (Age range 26-51), mean weight 94.6±18.9 kg (Weight range 75.7-113.5 kg), and mean BMI is 34.4±5.3 kg/m2 (Body Mass Index range is 29.1-39.7 kg/m2). Triglycerides are 145.1 ± 62.8 mg/dL (82.3-207.9 mg/dL), and LDL cholesterol is 133.1 ± 48.1 mg/dL (85-181.2 mg/dL), while HbA1c is 5.1 ± 1.1 (4-6, was in the range of 2). (Table 1).
Table 1
Patient profile before Elipse Balloon treatment
| Number of patients | 1770 |
| Gender (F/M) | 1264/506 |
| Age (Year) | 38.8 ± 12 |
| Weight(kg) | 94.6 ± 18.9 |
| BMI (kg/m2) | 34.4 ± 5.3 |
| Triglyceride (mg/dL) | 145.1 ± 62.8 |
| LDL Cholesterol (mg/dL) | 133.1 ± 48.1 |
| HbA1c (%) | %5.1 ± 1.1 |
During insertion, 99.9% of patients were able to swallow the balloon, and 35.9% required stylet assistance (Table 2). Eleven (0.6%) patients vomited the deflated balloon at the end of the normal treatment period. This was an uncommon method of balloon exit and was not associated with any adverse events.
Table 2
Balloon placement and passage performance
| Placement | |
| Swallowed | 1133 (%64.0) |
| Swallowed with stylet assistance | 636 (%35.9) |
| Placement failed | 1 (%0.06) |
| Balloon passage | |
| Stool | 1692 (%95.6) |
| Vomited balloon | 11 (%0.6) |
| Endoscopic removals (all causes) | 63 (%3.6) |
| Surgical removals | 4 (%0.02) |
Fifty-two (2.9%) patients had intolerance requiring early endoscopic balloon removal. Eleven (0.6%) balloons deflated prematurely and were expelled by normal means. Four (0.2%) balloons were removed endoscopically after it was discovered that these patients had previously had surgery that was contraindicated (not suitable for balloon therapy). Three (0.17%) small bowel obstructions occurred that required laparoscopic surgical removal of the balloon. However, these three balloons consisted of an earlier design generation of the balloon, which was manufactured early in the study (2016). Four (0.02%) events of spontaneous overinflation of the balloons occurred. Esophagitis developed in one patient (0.06%) and pancreatitis in one patient (0.06%), both of which required removal of the endoscopic balloon. One (0.06%) patient had gastric perforation that required laparoscopic surgical repair and removal of the Elipse balloon. A gastric dilatation (0.06%) occurred 15 days after placement of the ellipse balloon. This was resolved by switching the patient from a solid diet to a liquid diet for 48 hours. In addition, there was 1 (0.06%) case of gastric outlet obstruction requiring endoscopic removal of the balloon and 1 (0.06%) case with delayed intestinal transit (Table 3). There were no thromboembolic complications and deaths in the study.
Table 3
Adverse events and complications
| Intolerance requiring endoscopic removal | 52 (%2.9) |
| Early deflation (< 3 months) | 11 (%0.6) |
| Spontaneous hyperinflation | 4 (%0.2) |
| Small bowel obstruction (first balloon design) | 3 (%0.17) |
| Gastric dilation | 1 (%0.06) |
| Esophagitis | 1 (%0.06) |
| Pancreatitis | 1 (%0.06) |
| Gastric perforation | 1 (%0.06) |
| Delayed intestinal transit | 1 (%0.06) |
| Gastric outlet obstruction | 1 (%0.06) |
4 months after elipse swallowable gastric balloon application, overall mean weight loss (WL) was 13.5±5.8 kg, mean percent excess weight loss (%EWL) was 67.0±64.1, and mean BMI reduction (BMIL) was 4.9± It was ±2.0 points. The percentage of total body weight loss (TBWL%) was 14.2± 5.0 (Table 4). Elipse gastric balloon treatment led to improvements in all metabolic parameters investigated (Table 5).
Table 4
Weight loss results after Elipse treatment
| Weight Loss (WL) (kg) | 13.5 ± 5.8 |
| %TBWL (Percent Total Body Weight Loss) | 14.2 ± 5.0 |
| %EWL (Percent Excess Weight Loss) | 67.0 ± 64.1 |
| BMIL (kg/m2) (Body Mass Index Loss) | 4.9 ± 2.0 |
Table 5
Metabolic data results
| Beginnig | After 4 Months | |
| Triglyceride (mg/dL) | 145.1 ± 62.8 | 99.4 ± 21.8 |
| LDL (mg/dL) | 133.1 ± 48.1 | 106.9 ± 27.9 |
| HbA1c (%) | 5.1 ± 1.1 | 4.8 ± 0.8 |
Endoscopic Gastric balloons offered a less invasive (side effect-risk) alternative to surgical treatments for overweight and obese patients. Although drug therapy is more effective than diet and exercise, the application of endoscopic balloons has been limited due to the need for endoscopy for insertion and removal [12]. It is recommended in morbidly obese patients as a less invasive treatment than bariatric surgery [13, 14] and to reduce comorbidities and surgical risk [15, 16]. Commercially available intragastric balloons have all been shown to have comparable weight loss results, although slightly different [1-3]. Several studies in recent years have shown that data on both the efficacy and safety of the Elipse swallowable gastric balloon compare very favorably with other, longer duration balloons [1–3, 17, 18]. The Orbera balloon, the most widely used endoscopically inserted intragastric balloon, is the closest in size, shape and function to the Elipse balloon. The largest analysis by Orbera [19] shows that Orbera’s early balloon removal rate is 7.5%.
This current study is the largest study of the Elipse gastric balloon since its introduction. It is also one of the largest gastric balloon studies ever done. The centers included in the study are high-volume obesity treatment centers located in many different countries in Europe and the Middle East. This has provided a geographically and demographically diverse study population. The study shows an average TBWL of 14.2% and a BMI change of 4.9 points in just 4 months of balloon use. These results are in line with existing research showing that 80–90% of weight loss from a 6-month balloon occurs in the first 3 to 4 months after balloon insertion, followed by weight loss plateaus [20, 21]. Interestingly, according to previously established data, patients treated with the Elipse balloon program (including scales and smartphone app) maintained 72% of their weight loss 12 months after balloon expulsion [5]. This suggests that this may be associated with persistent and strong physiological changes during treatment, maintenance of lifestyle changes induced by continued use of the scale and applicator, or a combination of both.
Results include ease of insertion, balloon safety and performance, and complications, as well as the effect of the Elipse gastric balloon on weight and metabolic parameters. Overall efficacy results are in line with those reported in previous, smaller Elipse balloon studies. (Table 6). In addition, the entire Elipse weight loss program, including balloon, scale and smartphone application, was used in this study. Having these digital tools can create synergy to increase weight loss during the 4-month balloon period and also help maintain weight loss after balloon burst. All other patients completed the program, except for 63 patients (3.6%) who were unable to complete the program due to intolerance or other adverse events. This high follow-up rate has been achieved thanks to the close communication between the patient and the physician team (with the support of wireless scale and smartphone application) and has increased the quality of follow-up.
Table 6
Average weight loss outcomes on Elipse treatment in published studies
| Sample Size (n) | Weight Loss (kg) | BMI loss (kg/m2) | TBWL% | EWL% | |
| Current Study (2019) | 1770 | 13.5 ± 5.8 | 4.9 ± 2.0 | 14.2 ± 5.0 | 67.0 ± 64.1 |
| Jamal et al. [5]* (2019) | 106 | N/A** | 3.7 | 10.9 | N/A** |
| Alsabah et al. [4] (2018) | 135 | 13.1 ± 6.1 | 4.9 ± 2.2 | 15.1 ± 9.5 | N/A** |
| Raftopoulus et al. [3] (2017) | 12 | 15.4 | 5.4 | 14.6 | 50.2 |
| Machytka et al. [1] (2016) | 34 | N/A** | 3.9 ± 3.1 | 10 ± 6.6 | N/A** |
| Genco et al. [2] (2017) | 38 | 12.7 | 4.2 | 11.6 | 26 |
*Values reported are at 3rd and 6th month, because there are no data at 4th month
**Data were reported as initial and final and not in reduction
Subgroup efficacy analysis demonstrates that the %TBWL was similar in the overweight (BMI < 30 kg/m ), obese (BMI 30–40 kg/m ), and super‑obese (BMI > 40 kg/m ) populations: 13.3%, 14.4%, and 14.7% respectively (Table 7). Results also were similar in males and females (Table 8).
Tablo 7
BMI and % TBWL
| BMI | % TBWL |
| < 30 (patient number 302) | 13.3 ± 4.7 |
| 30–40 (patient number 1230) | 14.4 ± 4.9 |
| > 40 (patient number 196) | 14.7 ± 4.2 |
Table 8
%TBWL on males and females
| Gender | % TBWL |
| Male | 13.8 ± 5.2 |
| Female | 14.4 ± 5.0 |
Elipse gastric balloon was easily swallowed except for only one patient. 35.9% of patients needed stylet assistance to assist swallowing. Ease of the insertion procedure and elimination of endoscopy are the two main advantages of the Elipse balloon. These aspects were positively received by both physicians and patients. Post-procedure patient comfort is provided by the combination of treatment containing ondansetron and aprepitant given before and after the Elipse balloon application, which reduces complaints such as nausea and vomiting. The early removal rate due to intolerance due to the elipse balloon is low with 2.9%. In this study, 95.6% of the balloons passed through the gastrointestinal tract safely and were excreted with feces. Eleven empty balloons (0.6%) vomited at the end of their residence time without any adverse effects. Serious adverse events were very rare and included 3 small bowel obstructions treated laparoscopically. However, these occurred in an earlier generation of the Elipse balloon, and no other small bowel obstructions were reported in the 635 patients treated with the next-generation balloon as of 2018, following design changes to mitigate this condition. Spontaneous swelling is a familiar phenomenon in all liquid filled balloons. In this study, four patients (0.2%) presented with mild to moderate symptoms of intolerance and spontaneous hyperinflation on imaging. These balloons were removed endoscopically without complications. A study identified the root cause of hyperinflation resulting in a production change in the fill fluid and reduced other events in this study.
Metabolic data on metabolic disorders associated with obesity were collected, and improvement was observed in all three measured metabolic parameters. (LDL, triglycerides and HbA1c). (Table 5)
Main Article: The Procedureless Elipse Gastric Balloon Program: Multicenter Experience in 1770 Consecutive Patients



















Les informations, images et commentaires sur les opérations chirurgicales mentionnés sur ce site sont à titre informatif uniquement. La décision sur le diagnostic, le traitement et les modalités de suivi sera prise par le médecin.